I have a passion for developing and marketing innovative products that increase procedural efficiency and improve patient outcomes. The medical device industry has allowed me to seamlessly merge my medical education, clinical experience, and knowledge gained over the past 11 years leading engineering and product development teams through all phases of product development and FDA submission, to launch of over 75 spinal implant and surgical instrument product lines.
Recently, I’ve lead research, product development, and marketing initiatives for a novel titanium material that mimics the complex porous architecture of bone by combining additive manufacturing (High definition 3-D Printing) with a bioactive nanotechnology surface for superior implant integration and decreased post-operative infection risk.
What are NanoSmart Devices?
NanoSmart Devices are a new breed of implantable titanium materials that participate in the healing process.
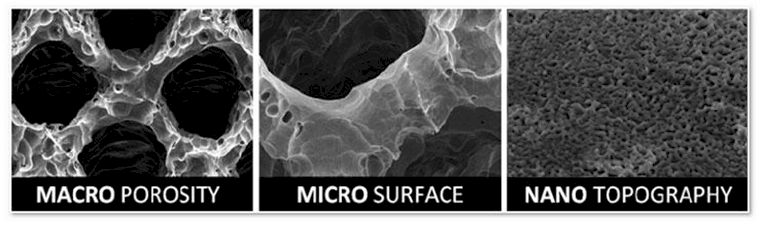
Nano-sized surface features change the behavioral characteristics of the underlying titanium material to accelerate implant integration into host bone and decrease bacterial colonization to reduce risk of post-operative infection.
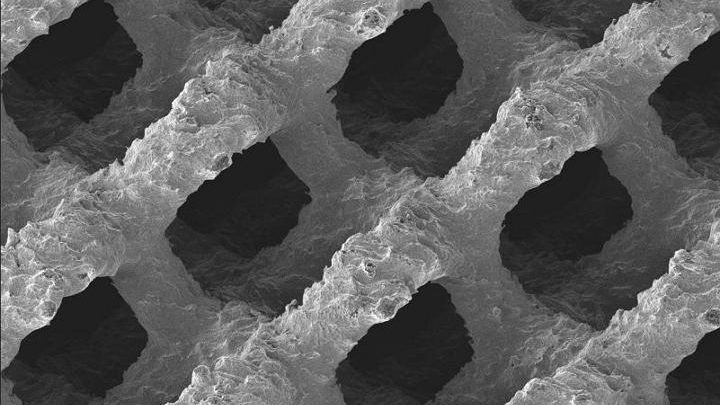
NanoSmart Devices are manufactured to exacting specifications utilizing high definition 3-D printing technology to replicate the porous cellular architecture of cancellous bone.
This new manufacturing process makes it possible to create any three dimensional complex structure or geometry that cannot be created by traditional orthopedic manufacturing processes.
Highly interconnected 300, 500 & 700µm pores create an ideal environment for natural influx of proteins, hormones, growth factors and mesenchymal stem cells to promote osteoinduction.
The uniform 3-D cellular architecture, with a consistent 70% porosity, provides an optimal biomechanical and biological environment for promotion of osseous tissue regeneration throughout entire device prior to encapsulation.
Compared to smooth implant surfaces, nano surfaced titanium surfaces have demonstrated a statistically significant reduction in bacterial colonization.
Pre-Clinical Areas of Study for NanoSmart Research Included:
- Osteoconduction and osteoinduction validation studies
- Nanotechnology claims
- Debris impaction results
- Bacterial adherence / bacterial colonization study
- Surface energy validation
- Modulus of elasticity validation
- Frictional coefficient analysis
- Injectable material flow analysis & instrument design
- Imaging characteristics – scatter artifact, assessment of fusion mass
- Subsidence study
- Therapeutic impregnation or coating investigation (Drug elution)
- Critical defect study (animal) – (traditional & solid designs)
- Intervertebral fusion study (animal)- (traditional & solid designs)
- Comparative Effectiveness Research: Multi-center fusion outcome study versus Allograft and/or PEEK
- Disintegrin Polymer Technology